CBL
What is CBL? In this chapter, we discuss this unknown minor cannabinoid, its effects, and whether it has any medical benefits.

What is CBL?
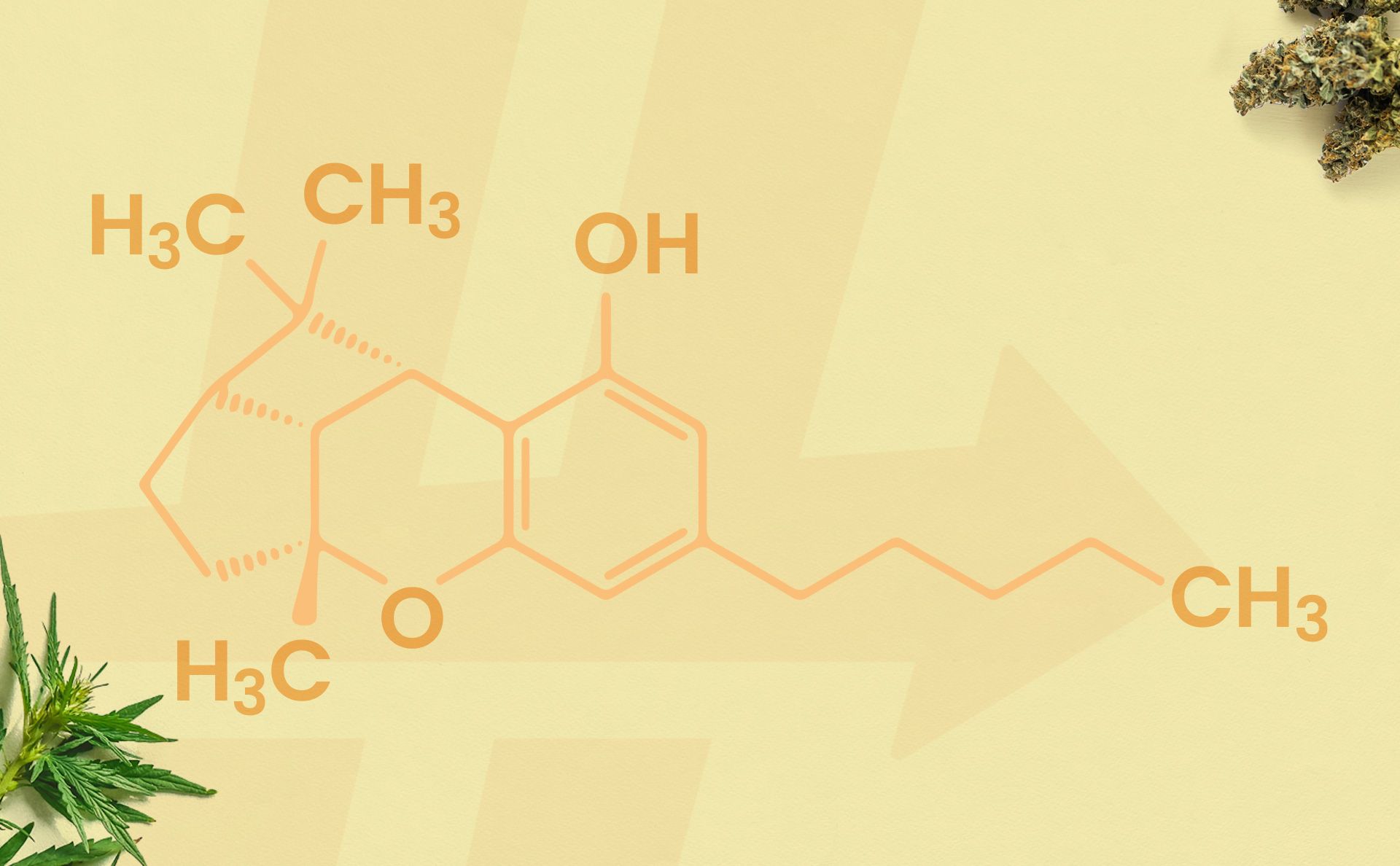
CBL, or cannabicyclol, is an ultra-rare cannabinoid found in the cannabis plant. It is an ancient cannabinoid that was found in a 2,700-year-old Chinese shaman grave, due to its remarkable stability. However, CBL was first technically discovered in 1964.
Similar to other minor cannabinoids like CBN, CBL forms during the degradation process of the cannabinoid cannabichromene (CBC). This process occurs when cannabis is exposed to UV light, heat, or air over time. Since it is only found in minute amounts, researchers must synthesize CBL for study – and it is not appreciably found in retail products nor sold as an isolate.
Lack of research, consumer awareness, and demand for this kind of cannabinoid means that you probably won't find any CBL products anytime soon. However, the little that we do know about CBL generally fits in with some of the other key features we know about more familiar cannabinoids.
Let's dive into it.
Effects of CBL
CBL does not have any impairing effects because it lacks the characteristic double bond that THC has.
However, CBL most likely interacts with the endocannabinoid system in ways other than through CB1 receptors, such as through CB2 or endocannabinoid system enzymes (e.g., FAAH, MAGL, etc.)
As such, it may likely share similar side effects to other non-imparting cannabinoids like CBC or CBD. But there aren't any clinical studies to know for sure.
We also don't know yet if CBL targets any other receptors in our brains that influence our mood or anxiety. If it does, it likely has a very weak effect because it’s largely inactivated by oxidation.
Potential Benefits of CBL
There is little known about this cannabinoid, but it likely shares similar much weaker potential benefits and effects as its closest parent, cannabichromene.
Research shows us that some of the potential effects of CBL include:
- Anti-inflammatory properties – CBL is the weakest anti-inflammatory cannabinoid studied, with little to no activity compared to CBN, CBDA, THC and isomers, CBD, and CBC
- Anticancer and antiproliferative activity demonstrated in test tubes on human colon cancer cells
- Weak antibacterial potential, however not against MRSA
As an aside, there was a flawed study conducted in 1976 on inbred rabbits given CBL by IV jugular. Sparing the gruesome details, suffice to say that the experiment is unreliable and the faulty conclusions drawn (e.g., proconvulsant, coagulant) are the opposite of what we find from cannabinoids and should not truly be applied to CBL. This is important to note in case you come across this study elsewhere.

What the expert says...
Dr. Abraham Benavides
"CBL forms during the degradation process of the cannabinoid CBC, to which it likely shares weaker potential effects. This oxidative process occurs when cannabis is exposed to UV light, heat, or air."
What We Learned: CBL
Learning about CBL is an important part of better understanding cannabis science. Here’s what we learned in the CBL chapter:
- CBL, or cannabicyclol, is an ultra-rare cannabinoid.
- CBL forms during the degradation process of the cannabinoid CBC. This process occurs when cannabis is exposed to UV light, heat, or air.
- CBL is non-impairing, meaning it does not get you high.
- So far CBL demonstrates very weak, if any, activity as an anti-inflammatory, anti-cancer, and antimicrobial agent.
- CBL is not a commercially available cannabinoid. It is only synthesized for research purposes
Thankfully, research on minor cannabinoids is slowly expanding, and along with it, we will eventually gain a better understanding of the potential medicinal properties and effects of cannabicyclol.
Reaching closer to the finish line! Answer the below question and keep moving along in this guide.
Citations
- Burstein, S., Levin, E., & Varanelli, C. (1973). Prostaglandins and cannabis—II inhibition of biosynthesis by the naturally occurring cannabinoids. Biochemical Pharmacology, 22(22), 2905–2910.https://doi.org/10.1016/0006-2952(73)90158-5
- Cerino, P., Buonerba, C., Cannazza, G., D’Auria, J., Ottoni, E., Fulgione, A., Di Stasio, A., Pierri, B., & Gallo, A. (2021). A Review of Hemp as Food and Nutritional Supplement. Cannabis and Cannabinoid Research, 6(1), 19–27.https://doi.org/10.1089/can.2020.0001
- Crombie, L., Ponsford, R., Shani, A., Yagnitinsky, B., & Mechoulam, R. (1968). Hashish components. Photochemical production of cannabicyclol from cannabichromene. Tetrahedron Letters, 55, 5771–5772.https://doi.org/10.1016/s0040-4039(00)76346-5
- Filer, C. N. (2022). Cannabinoid Photochemistry: An Underexplored Opportunity. Cannabis and Cannabinoid Research, 7(6), 725–727.https://doi.org/10.1089/can.2022.0113
- Goldman, S., Bramante, J., Vrdoljak, G., Guo, W., Wang, Y., Marjanovic, O., Orlowicz, S., Di Lorenzo, R., & Noestheden, M. (2021). The analytical landscape of cannabis compliance testing. Journal of Liquid Chromatography & Related Technologies, 44(9–10), https://doi.org/10.1080/10826076.2021.1996390
- Klahn, P. (2020). Cannabinoids-Promising Antimicrobial Drugs or Intoxicants with Benefits? Antibiotics, 9(6), 297.https://doi.org/10.3390/antibiotics9060297
- Korte, F., & Sieper, H. (1964). [ON THE CHEMICAL CLASSIFICATION OF PLANTS. XXV. QUANTITATIVE DETERMINATION OF HASHISH COMPONENTS BY THIN-LAYER CHROMATOGRAPHY]. Journal of Chromatography, 14, 178–183.https://doi.org/10.1016/s0021-9673(00)86608-x
- Lee, H.-S., Tamia, G., Song, H.-J., Amarakoon, D., Wei, C.-I., & Lee, S.-H. (2022). Cannabidiol exerts anti-proliferative activity via a cannabinoid receptor 2-dependent mechanism in human colorectal cancer cells. International Immunopharmacology, 108, 10https://doi.org/10.1016/j.intimp.2022.108865
- Martin, P., & Consroe, P. (1976). Cannabinoid induced behavioral convulsions in rabbits. Science (New York, N.Y.), 194(4268), 965–967.https://doi.org/10.1126/science.982057
- Russo, E. B., Jiang, H.-E., Li, X., Sutton, A., Carboni, A., del Bianco, F., Mandolino, G., Potter, D. J., Zhao, Y.-X., Bera, S., Zhang, Y.-B., Lü, E.-G., Ferguson, D. K., Hueber, F., Zhao, L.-C., Liu, C.-J., Wang, Y.-F., & Li, C.-S. (2008). Phytochemicalhttps://doi.org/10.1093/jxb/ern260
- Vree, T. B., Breimer, D. D., van Ginneken, C. A. M., & van Rossum, J. M. (1972). Identification of cannabicyclol with a pentyl or propyl side-chain by means of combined as chromatography—Mass spectrometry. Journal of Chromatography A, 74(1), 124–127.https://doi.org/10.1016/S0021-9673(01)94980-5
- Yeom, H.-S., Li, H., Tang, Y., & Hsung, R. P. (2013). Total Syntheses of Cannabicyclol, Clusiacyclol A and B, Iso-Eriobrucinol A and B, and Eriobrucinol. Organic Letters, 15(12), 3130–3133.https://doi.org/10.1021/ol401335u
Test your knowledge, track your progress and earn your badge.
CBL has impairing effects.

Dr. Abraham Benavides
Dr. Abraham Benavides is an internationally-recognized cannabis research expert, experienced medical advisor, and full-tuition merit scholar of the George Washington University School of Medicine and Health Sciences. Dr. Abe enjoys helping patients as a writer, educator, and cannabis health coach at the GW Center for Integrative Medicine.